UNCONFINED COMPRESSION TESTING OF COLLAGENASE-DIGESTED PORCINE ARTICULAR CARTILAGE
LAST UPDATED: 08/22/2017 by Zack Berent
I. Lab Logistics and Location
II. Motivation for the lab
III. Background
IV. Relevant Literature
V. Pre-lab Assignment
VI. Methods
VII. Analysis of Results and Discussion of Findings
VIII. Directions for the Teaching Assistant
I. Lab Logistics and Location
This lab requires three meetings. The first meeting will be a lecture in the usual Friday classroom during the Monday-Wednesday lecture time, covering compression testing and animal dissection.
For the second and third meetings, students will meet during their scheduled lab time, either for the full period or part of the period.
For the second meeting, students will meet during their scheduled lab time for the full 60 minute period. We will meet in Prof. Wagoner Johnson’s lab space (MEB 111) where the sample prep demonstration will be conducted. During this meeting, students will observe and participate in sample preparation procedures required for conducting compression tests of soft biological materials, such as articular cartilage. Starting from the bulk knee joint sample, the TA will demonstrate how to core cartilage plugs that are amenable for compression tests.
For the third meeting, students will meet for only 30 minutes of their scheduled lab time. We will meet in the first floor lobby of the Institute for Genomic Biology. This is the building with play-doh like structures, and it is directly across from the Morrow Plots. Enter through the doors facing the play-doh like structures. Since we will only meet for 30 minutes, the TA will split the class in half; let the TA know of preferences (e.g. first 30 minutes, prefer same time as lab partner). Note that IGB is a 10 minute walk from MEB and you CANNOT be late to IGB or else you will get locked out. If you have class or a meeting before or after your lab time, let your TA know your preference so that you have sufficient time to make it to class and your meeting. The third meeting provides an opportunity for the students to observe first-hand and participate in compression tests of articular cartilage. Each lab group will generate a data file to contribute to the class data set. The TA is responsible for pooling data together and disseminating the file electronically.
Lab Write Up Policy
This lab may be written individually or in pairs. Lab reports will adhere to the normal 2-page extended abstract style report, see Lab Resources for more information. Your references can be listed on an additional page.
II. Motivation for the Lab
Osteoarthritis is a pathological condition where articular cartilage in joints breaks down or wears away, causing joint pain and stiffness, especially in the hands, neck, hips, lower back, and knees. Osteoarthritis affects 27 million Americans over the age of 25. In this lab, osteoarthritis of varying severity is simulated by digesting articular cartilage samples from pigs in different concentrations of collagenase, an enzyme that breaks down collagen in articular cartilage. Our aim is to investigate the collagenase-induced changes in the biomechanical properties of articular cartilage using compressive loads so as to better understand how osteoarthritis affects the mechanical properties of articular cartilage.
III. Background
Human Knee Anatomy
The overall anatomy of the human knee is similar to the anatomy of the porcine, or pig, knee. Specifically, observe the positions the medial and lateral condyles relative to the fibula and tibia as these are the locations from where we will be harvesting our cylindrical cartilage samples.
Image Credit: http://www.pinnacle-ortho.com
IV. Relevant Literature
Recommended Reading
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. Journal of Orthopaedic Research 2001; 19(6) 1113-1121.
V. Pre-lab Assignment
The pre-lab assignment is due at the second meeting. Submit the pre-lab assignment to Compass by 11:59 PM of the Friday of Sample Prep. Components of the pre-lab assignment must be done individually; some components of the lab may be done individually or as part of a pair.
VI. Methods
Sample Procurement and Storage
Adult porcine knee samples are obtained from the Meat Science Laboratory at the University of Illinois at Urbana-Champaign. Tissue is stored at -20°C until cylindrical tissue samples that are amenable for compression testing can be prepared.
Sample Preparation
Cylindrical cartilage testing samples will be excised from the lateral and medial condyles of the knee joint using a 7mm-diameter trephine drill bit mounted on Dremel 8200 tool mounted on a Dremel 220 Workstation. Care will be exercised to limit the time between sample harvesting and compression testing so as to avoid any altered mechanical properties caused by environmental exposure ex vivo. After harvesting the samples, they will be stored at 5°C in PBS until the testing date.
Collagenase Treatment
Cored cylindrical cartilage samples will be divided into three groups:
- One-third of the cartilage samples will be tested without treatment – these are the control samples.
- One-third of the will be subjected to a “low concentration” (5U/mL) treatment.
- One-third will be subjected to a “high concentration” (50U/mL) treatment.
As purchased, collagenase is rated by its enzyme activity and comes in a powder-form. We will use Hanks’ Buffered Salt Solution (HBSS), a biocompatible solution, as our enzyme solvent. Knowing the enzyme activity, we calculate the mass of collagenase that we should dissolve into a volume of HBSS to achieve our desired concentrations. The volume of HBSS should be selected such that it is a reasonable volume to measure and the the mass of collagenase that is computed is also reasonable to measure. An example of this calculation is provided below.
Assume the enzyme activity for one unit of collagenase is 193U/mg. Knowing the enzyme activity, we calculate the mass of collagenase that we should dissolve into 50mL of HBSS to achieve our desired concentrations, as follows:
Cartilage samples (with the subchondral bone still attached) undergoing treatment will be incubated in the appropriate collagenase solutions for 24 hours at 37 degrees Celsius. Untreated samples will be also stored at the same temperature for the same time. Prior to compression testing the cartilage, the subchondral bone will be separated from the cartilage plug with a razor blade, and the diameter and thickness of sample will be measured for stress/strain calculations using a caliper. Samples will be loaded to 10% strain.
Testing Instrument
We use a Bose 5110 Biodynamic System (see picture below) housed in the Institute of Genomic Biology to perform our unconfined compression tests. Our input into this system is a displacement waveform consisting of an initial dwell time of 10 seconds, a compression segment at a rate of 0.01mm/s to achieve 10% strain, a dwell segment of 60 seconds maintaining the strain level at 10%, and finally unloading at a rate of 0.01mm/s.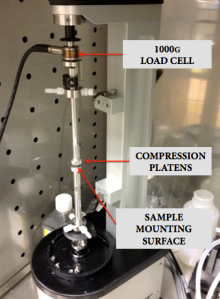
Maintaining sample hydration during compressive testing is crucial for biological samples. Although this instrument enables us to perform compressive tests while samples are fully immersed in a biocompatible solution (such as PBS), we opt to test samples in air due to time constraints. Due to the short nature of tests, we assume that sample hydration will not be an influential factor.
VII. Analysis of Results and Discussion of Findings
Data
Load, displacement, and time data will be collected during testing and converted into stress-strain data.
Report Guidelines
A class-wide data set will be provided. As individual students or in pairs, you will submit an extended abstract style report for this lab, see Lab Resources for more details. In writing your report, you must:
- Perform a literature search for similar experiments and provide a brief summary in the introduction section of your lab report. Your introduction should relate to both the biology and the mechanics of the cartilage. Please cite 2-3 papers to support your claims.
- Assess the data set to remove outlier data as deemed appropriate.
- Generate one plot containing one representative stress-strain curves for control, low, and high treatment groups on the same plot (i.e. 1 plot, 3 lines).
- Compute a Young’s modulus from the linear region for each sample (at least 5 samples from each group).
- Tabulate the average elastic modulus and the standard deviation for each treatment group.
- Use ANOVA to find statistically significant differences among the mean Young’s moduli for the three groups. Use an level of 0.05. If ANOVA is significant, use post-hoc analysis to find pair-wise differences.
- Discuss the importance of these findings, including limitations or potential sources of error, and future directions, within the discussion section.
- Follow all other lab report guidelines.
VIII. DIRECTIONS FOR THE TEACHING ASSISTANT
The information below is intended primarily for the lab teaching assistant.
Biosafety
To ensure safe handling and disposal of animal tissue, it is recommended that the TA successfully complete the Understanding Biosafety training module from the Division of Animal Research at the University of Illinois at Urbana-Champaign.
Sample Disposal
Samples post-testing and all other biological waste that is generated should be discarded in accordance with the policies detailed by the Division of Research Safety (DRS) in the Treatment and Disposal of Biological Materials. To schedule pickup of pathological waste for incineration, the TA should go to http://www.drs.illinois.edu/biowaste. The TA should ensure that a completed DRS Incineration Tag is securely attached to the handle of each bag. Refrigerate or freeze waste between disposal and pickup.
Requisite TA Training
- The TA should receive training on the Bose 5110 Biodynamic System and gain experience on the instrument by testing phantom samples, such as hydrogels.
- The TA should become comfortable with the dissection of the porcine knee joint and its basic anatomy and gain experience using the band saw and scalpels as is necessary for dissection.
- The TA should become familiar with operation of the trephine drill mounted on the Dremel 8200 tool. It is advised that the first-time TA practice coring cartilage plugs prior to conducting the sample preparation demo in order to find a technique that best suits him or her.
- The TA should ensure that the Bill of Materials is fulfilled prior to beginning this lab.
Bill of Materials
Porcine Hind Legs with Knee Joint
Source: Meat Sciences Lab at the University of Illinois at Urbana-Champaign
To provide the Meat Sciences Lab sufficient lead time, it is advised that the TA contact Benjamin Peterson [bcpeters AT illinois DOT edu] at least 4 weeks in advance of the lab start date. For each lab section, with about 10-15 students, 2 legs are recommended.
Collagenase from Clostridium histolyticum (C0130-100MG from Sigma Aldrich)
Source: Sigma Aldrich
Hanks’ Buffered Saline Solution 1X
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Scalpel Disposable #11
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Latex/Nitrile Disposable Gloves
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Bench Paper (Green)
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Tubes (50mL) Corning Centrifuge
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Razor Blades Single Edge
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
6.0/7.0 mm Internal/External Long Trephine Drills
Source: Osseous Technologies of America
Dremel 8200 Tool/Dremel 220 Workstation
Source: Mechanical Engineering Building, Room 111/113
Digital Calipers
Source: Mechanical Engineering Building, Room 111/113
-20C Freezer
Source: Mechanical Engineering Building, Room 111/113
Analytical Lab Scale
Source: Mechanical Engineering Building, Room 111/113 or CORE/Institute of Genomic Biology
Band Saw
Source: Mechanical Engineering Building, Room 111/113
37C Incubator
Source: CORE/Institute of Genomic Biology
Lab Utensils and Materials
Source: Mechanical Engineering Building, Room 111/113 or Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
a. Dissecting Forceps
b. Lab Spoon
c. Tapered Micro Spatula
d. Weighing Paper
e. Graduated Cylinders
f. Biohazard Trash Bags and DRS Incineration Tags
g. Surface Disinfectant (70% Isopropyl Alcohol)