FOURIER-TRANSFORM SECOND HARMONIC GENERATION IMAGING OF PORCINE TENDON
LAST UPDATED: 10/7/2019 by Amir Ostadi Moghaddam
I. Lab Logistics and Location
II. Motivation for the lab
III. Background
IV. Relevant Literature
V. Pre-lab Assignment
VI. Methods
VII. Analysis of Results and Discussion of Findings
VIII. Directions for the Teaching Assistant
I. Lab Logistics and Location
The second lab requires three meetings. The first meeting will be a lecture in the usual Friday classroom during the Monday-Wednesday lecture time, covering background information about the lab. The TA will present on the Fourier-Transform Second Harmonic Generation imaging technique.
For the second meeting, students will meet for only 30 minutes of their scheduled lab time. We will meet in the first floor lobby of the Institute for Genomic Biology, as with the first lab. This time, we will be going downstairs to the Core Facilities. During this meeting, the TA will introduce students to the sample preparation methodology for Second Harmonic Generation imaging.
For the third meeting, students will meet for 30 minutes of their scheduled lab time. We will meet in the first floor lobby of the Institute for Genomic Biology, similar to the second meeting. The TA will lead an interactive session demonstrating real-time Second Harmonic Generating imaging on porcine tendon.
Lab Write Up Policy
This lab may be written individually or in pairs. Lab reports will adhere to the normal 2-page extended abstract style report, see Piazza for more information. Your references can be listed on an additional page.
II. Motivation for the Lab
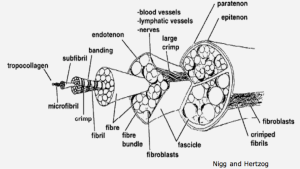
Tendon connects muscle and bone using bundles of parallel collagen fibers. The highly organized, parallel fibers provide mechanical strength in tension. Tendonitis, or inflammation of the tendon, is commonly caused by repetitious movements, and thus affects many athletes. For example, basketball players commonly have Achilles tendonitis from repetitive jumping, and tennis elbow is a common type of tendonitis for tennis players.
III. Background
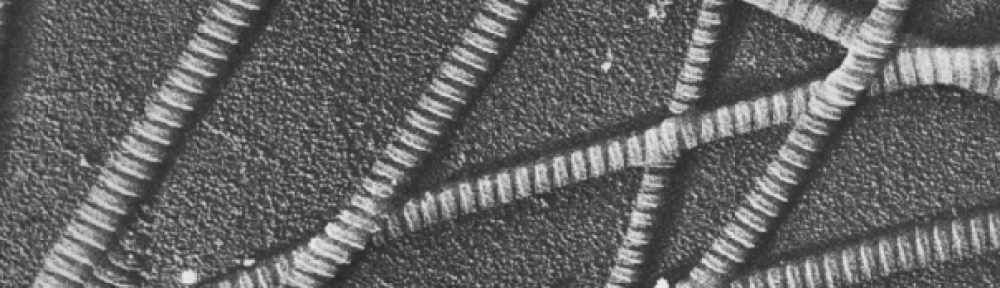
In inflamed tendon, collagen fiber organization is disturbed and the fibers no longer properly link together, thus the mechanical strength of the tendon decreases. This disruption may be simulated using collagenase.
Second harmonic generation (SHG) is a nonlinear optical process used to analyze structural changes. SHG provides three-dimensional resolution images with high contrast and does not require staining. Fourier-Transform SHG creates a quantitative measure, which leads to its use in diagnostics. See Sivaguru et al. [2010] for more information. In this lab, we combine SHG images of tendon tissue with spatial harmonic analysis (using Fourier transforms) to quantitatively analyze the collagen architecture of this tissue.
Studying the orientation of the collagen architecture in tendon, as well as other tissue, is of significance because it will enable the development of a reliable diagnostic tool, provide input and validation parameters for computational models, and will undoubtedly advance the field of tissue mechanics. Furthermore, from in-class discussions, you should also recall that the collagen architecture is generally important for studying biological tissue because it provides information about the type of loading the tissue withstands in vivo, and it can also help us differentiate healthy tissue from injured/diseased tissue in a quantitative way.
IV. Relevant Literature
Pre-Lab Reading for Second Harmonic Generation (SHG) Microscopy
The concepts in these readings are important to the Fourier-Transform Second Harmonic Generation technique are more advanced compared to the concepts discussed in our other labs. These papers will be useful in answering the pre-lab assignment.
The following reading is required.
- Y. Lau, H.K. Sangha, E.K. Chien, B.L. McFarlin, A.J. Wagoner Johnson, and K.C. Toussaint, “Application of Fourier transform-second-harmonic generation imaging to the rat cervix,” Journal of Microscopy 251, 77-83 (2013)
The following readings are recommended.
- Ambekar, M. Chittenden, I. Jasiuk, and K. C. Toussaint, Jr., “Quantitative second-harmonic generation microscopy for imaging porcine cortical bone: Comparison to SEM and its potential to investigate age-related changes,” Bone 50, 643-650 (2012).
- Sivaguru, S. Durgam, R. Ambekar, D. Luedtke, G. Fried, A. Stewart, and K. C. Toussaint, Jr., “Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging,” Optics Express 18, 24983-24993 (2010).
V. Pre-lab Assignment
The pre-lab assignment is due at the second meeting. Submit the pre-lab assignment to Compass by 11:59 PM of the Friday of Testing. Components of the pre-lab assignment must be done individually; some components of the lab may be done individually or as part of a pair.
For this lab, we will be using ImageJ. You are required to complete the ImageJ tutorial using a provided image of a rat cervix as part of the pre-lab assignment.
VI. Methods
Sample Preparation
Porcine extensor digitorum lateralis tendon tissue was isolated in a similar fashion to Lab 1. The control tendon was stored in PBS and the collagenase-digested tendon was stored in 50 U/ml collagenase for 24 hours (See Lab 1 for more details). The specimen were then placed in cryomolds and filled with an optical cutting temperature (OCT) compound and frozen until before slicing. Using a Leica CM3050 S Cryostat, 8, 10, and 20 micron thick sections were cut. Both transverse and longitudinal cross-sections were cut for each specimen. Specimen were rinsed with PBS and mounted between two coverslips. A PowerPoint uploaded to Piazza will show the process.
Imaging
FT-SHG microscopy was performed on the Zeiss 710 microscope. Lab lecture will provide more information.
VII. Analysis of Results and Discussion of Findings
Lab Report Requirements
Data
SHG images of all 4 conditions (control transverse plane, control longitudinal plane, collagenase-digested transverse plane, collagenase-digested longitudinal plane) will be given as the output. You will perform analysis on the regions following the instructions provided in Piazza.
VIII. DIRECTIONS FOR THE TEACHING ASSISTANT
The information below is intended primarily for the lab teaching assistant.
Biosafety
To ensure safe handling and disposal of animal tissue, it is recommended that the TA successfully complete the Understanding Biosafety training module from the Division of Animal Research at the University of Illinois at Urbana-Champaign.
Sample Disposal
Samples post-testing and all other biological waste that is generated should be discarded in accordance with the policies detailed by the Division of Research Safety (DRS) in the Treatment and Disposal of Biological Materials. To schedule pickup of pathological waste for incineration, the TA should go to www.drs.illinois.edu/biowaste. The TA should ensure that a completed DRS Incineration Tag is securely attached to the handle of each bag. Refrigerate or freeze waste between disposal and pickup.
Requisite TA Training
- In order to instruct students about the sample preparation undertaken for SHG imaging, the TA should receive training on the Leica CM3050 cryostat instrument and gain experience on the instrument by slicing biological tissue.
- The TA should become comfortable with the concepts important to SHG imaging by reviewing appropriate literature, as well as by leveraging the expertise provided by personnel at the Institute for Genomic Biology.
- The TA should become familiar with operation of IMAGEJ as it relates to performing Fast Fourier Transforms.
- The TA should ensure that the “Bill of Materials” is fulfilled prior to beginning this lab.
Bill of Materials
Introductory Second Harmonic Generation Lecture
Source: Professor Kimani Toussaint
Professor Toussaint graciously provides the introductory lecture on FT-SHG microscopy to ME482 students. It is important that the TA contact him in advance of the beginning of the semester in order to schedule his lecture.
Second Harmonic Generation Imaging Sessions
Source: Dr. Sivaguru Mayandi
Dr. Sivaguru Mayandi has led SHG imaging sessions for students where he demoes live SHG imaging of rat cervix tissue. It is important that the TA contact him in advance of the beginning of the semester in order to schedule these imaging sessions. It is recommended that the TA also schedule a practice session with Dr. Mayandi.
Leica CM3050 Cryostat
Source: Institute for Genomic Biology
Instrument time should be reserved on the cryostat such that SHG sample preparation can be demoed to the students immediately before the SHG imaging sessions led by Dr. Mayandi. It is recommended that the TA also schedule a practice session on this instrument to become familiar with slicing and anatomy of the rat cervix. Slices should be 50 microns thick.
Rat Cervix Tissue
Source: Rat Breeder
Two to three rat cervix tissue samples should be allocated for this lab, but just one rat cervix tissue sample will suffice for a class of up to 30 students. Higher number is recommended since some samples may be utilized during practice sessions. Samples should be stored at -80 celsius until ready for use.
Rectangle No 1.5 Cover Slips (Size: ~60 x ~22mm)
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Square No 1.5 Cover Slips (Size: ~20 x ~30mm)
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Phosphate Buffered Saline Solution 1X
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Optimal Cutting Temperature (OCT) Compound
Source: Institute for Genomic Biology
Nail Polish Top Coat Clear
Source: Drugstore
Kimtech Science Kimwipes
Source: Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
Aqueous Tissue Mounting Medium
Source: Institute for Genomic Biology or Sigma Aldrich (C9368-30ML)
-80C Freezer
Source: Institute for Genomic Biology
Lab Utensils and Materials
Source: Institute for Genomic Biology or Life Sciences Storeroom at the University of Illinois at Urbana-Champaign
a. Fine-Tip Artist Paint Brushes (used to flatten and unwrinkle tissue on coverslip)
b. Biohazard Trash Bags and DRS Incineration Tags
c. Surface Disinfectant (70% Isopropyl Alcohol)